Equipment Description
This system consists of 4 fixed bed reactors. One reactor is full of the the nickel catalyst, while another is only 75% filled and the last two columns are 50% full. A pump pushes the fluid to flow through the each reactor. There is no heat exchanger needed to heat up the fluid. Instead, we use a large water bath which surrounds the reactors – a temperature-controlled jacket. The streams coming out of each reactor are collected in a collection tank found below each reactor.
Titrations will be conducted conducted during the experiment. In the fume cupboard besides – to the right – of the unit, you will find necessary chemicals and equipment needed to conduct the titrations safely. You will 50mL burettes with clamp stands; multiple 1mL, 5mL and 10mL pipettes with rubber safety bulbs. Stirring rods will also be available. In the cupboards below, you will find all the necessary beakers and flasks needed for your analysis.
Background & Theory
To review concepts behind the leaching process, click here.
Virtual tour of the operation unit
ZOOM IN AND OUT TO SEE THE CLOSER LOOK OF THE OPERATION UNIT. YOU CAN ALSO SELECT ANY OF THE FOLLOWING CLOSE-UP TOUR TO SEE VALVES AND MEASUREMENT DEVICES ON THE UNIT.
2D Photos


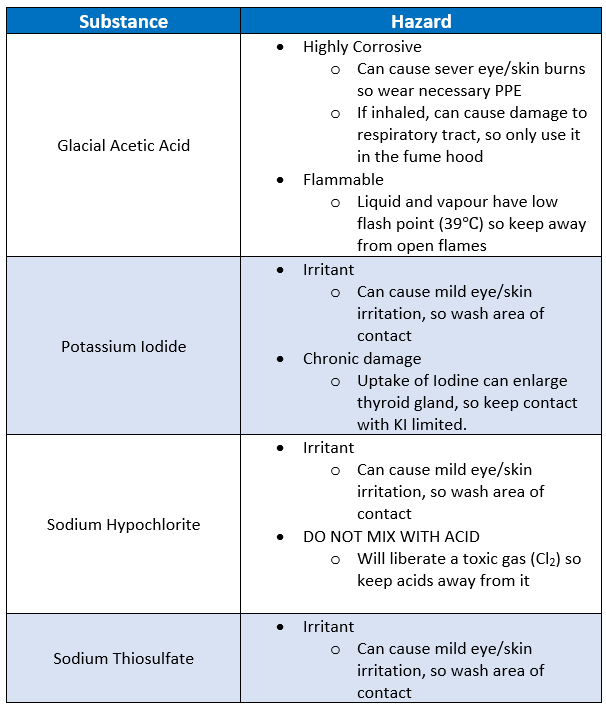
Safety Considerations
In this lab, you will be performing some analytical chemistry to determine analyze the effectiveness of our system. In doing so, please make sure to take care when performing this lab as the chemicals used here can cause serious damage without proper care:
-
Wear all necessary PPE (gloves, goggles, lab coat)
-
Preparations of standard solution should be done in a fume hood
-
Perform all titrations in a fume hood
-
If any acid is spilled, notify a TA, neutralize the spill with sodium bicarbonate, and clean up the spill
Summary of MSDS:
Procedure
I highly suggest before coming to the lab that the groups performing this lab should plan a system to complete the experiment with the time you have. Not only do you need to titrate to make the standard solutions, but we also need to perform a titration to analyze the bleach concentration coming out of the reactor. Furthermore, it’s normal to make a small mistake and then have to make even more of your standard solutions. On top of all this, the sample you collect from the reactor needs to be prepared before it can even be analyzed with a titration. In the time frame that you have, if you do not organize yourselves well, you will not be able to finish the lab in time, and therefore have little to no data for your lab report.
Preparation of Solutions
Preparing Starch Solution
-
Mix 0.5g of soluble starch in 5mL of cold water
-
Add 95mL of boiling water to the solution
-
Mix everything together and store in a sterilized container
Preparing Sodium Thiosulfate Standard Solution
-
Boil 1L of water and allow it to cool to a moderate temperature (50-70C)
-
Dissolve 25g of sodium thiosulfate crystals into solution
-
Weigh out 3.567g of potassium iodate and transfer this reagent to a 1L volumetric flask
-
Fill the flask up to the mark with water and dissolve the reagent thoroughly
-
Pipette a 50mL aliquot of the potassium iodate solution into a 250mL Erlenmeyer flask
-
Dilute the potassium iodate aliquot until the volume reaches 100mL
-
Dissolve 1g of potassium iodide into the solution created in step 6
-
After the potassium iodide dissolves, add 15mL of 1.0N (1 g/L) of hydrochloric acid
-
Immediately titrate the solution with our sodium thiosulfate solution
-
When the potassium iodate solution reaches a light-yellow colour, add 1mL (couple of drops) of starch solution
-
Continue the titration until the blue-black colour disappears and record the volume of the titrant
Operating Procedure
-
Place the feed suction tubing into the feed tank
-
Place the discharge tubing into a collection tank
-
Set the rpm setting of the pump and the temperature of the reactors
-
Turn on the pump and allow the feed to enter the reactor
-
At a specific time interval (recommended s 5 minutes), collect your samples and label them for analysis.
-
Once the system reaches steady state, you can stop this run.
-
Repeat the run at a different temperature, or a different rpm for the pump
Sample Analysis
-
Dissolve 2 to 3g of potassium iodide crystals in 50mL of water in a 250 Erlenmeyer flask
-
Add 10mL of acetic acid to the potassium iodide solution
-
Pipette a specific aliquot of the sample and dip the tip of the pipette into solution to add the sample to the solution
-
Immediately titrate with the standard sodium thiosulfate solution until the is a very pale yellow
-
Add a couple of drops of starch solution
-
Continue the titration until the blue-black colour disappears
-
Record the titre volume
Shutdown Checklist
-
Clean and rinse all glassware and dump the waste into their respective bins (e.g. inorganic waste in the green bin)
-
After cleaning, put all glassware back where they were found
-
Drain all liquid out of the reactors and into the discharge tank
-
Help the TA to remove and dump the discharge tank out
-
Turn off the water bath and pump